Stryker Implants
What is Stryker?
Global medical technology company founded in 1940
Fortune 500 company and revenues top $12 billion

Headquarters in Kalamazoo, Michigan
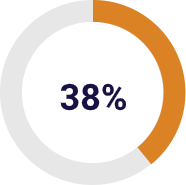
The orthopaedic division including knee, ankle, trauma and hip implants accounts for 38% of sales in 2017
Equipment can be found in 3,000 American hospitals
Recalled some of its products that led to injuries such as bone and tissue damage

Paid $97 million to the federal government to resolve criminal charges

1 CEO received a jail sentence

Over 3,400 people sued Stryker after problems with its Rejuvenate and ABG II hip implants led to recalls. Lawsuits were settled for $2 billion

Hip Replacements
Pending Lawsuits Against Stryker
Rejuvenate and AGB II Hip Implants

Number of Lawsuits
1,240 lawsuits pending in Minnesota federal court (MDL-2441) as of May 2018

Settlements
$1.43 billion to settle thousands of lawsuits in 2014; expanded in 2016 to over $2 billion

Patient Injuries
High levels of metal ions in blood and urine; bone and tissue damage; severe pain and revision surgery
LFIT V40 Femoral Head Products

Number of Lawsuits
2,271 lawsuits pending in Massachusetts federal court (MDL-2768) as of May 2018; lawsuits filed in New Jersey are consolidated as multicounty litigation (MCL)

Patient Injuries
Excessive wear and metallic debris; bone and tissue damage; severe pain; revision surgery and device failure, including disassociation of femoral head from hip stem and fractured hip stem trunnion
Most people who have hip replacement are between 50 and 80 yrs old.
Stryker recalls
2012
Product recalled – Rejuvenate and AGB II modular hip stems
Reason for recall – Elevated levels of the metals found in patients implanted with devices, causing bone and tissue destruction
> 50,000
units recalled

2016
Product recalled – LFIT Anatomic Cobalt-Chromium V40 femoral heads (LFIT V40)
Reason for recall – Small particles of the metal were released around a component in the hip device, resulting in erosion, bone and tissue damage, and device failure (components became disassociated from each other)

> 42,000
units recalled
NYU Langone Orthopedic Hospitals
5 implant failures associated with Stryker’s Tritanium cups implanted between 2011 and 2016.
5 implant failures
associated with Stryker’s Tritanium cups implanted between 2011 and 2016. The patients, who ranged from 49 to 68 years old, had all developed hip and groin pain within just a few months of their hip replacements. Subsequent imaging revealed the Tritanium components were loose.
A 2018 study
in the journal of Orthopaedic Proceedings found that more than a third of hips implanted with Tritanium shells demonstrated changes on X-rays within a year of surgery that could lead to “poorer clinical function.” The authors concluded that patients with these implants be closely monitored for signs of deterioration and component loosening.

The Accolade II
replaced the Accolade TMZF in the U.S. market. The Accolade II the latest product in the Accolade Hip System that has been implanted in more than 500,000 patients. A 2011 study analyzed 200 patients who received 214 Accolade TMZF implants. Five patients required revision surgery because of loosening, infection, instability or wear.

In July 2012,
Stryker voluntarily recalled all of its Rejuvenate and ABG II hip replacements from the market because of the potential for fretting and corrosion that could cause pain, swelling and tissue damage. The U.S. Food and Drug Administration classified the recall as Class II recalls and determined the cause of the recall to be “defective design.”
Thousands of people harmed by the hip implants sued Stryker because their implants failed and they had to have revision surgery. The company resolved many of the claims in a billion dollar settlement in 2014, but more than 1,000 lawsuits are still pending.
Stryker devices have caused a higher than normal occurrence of failure due to corrosion and fretting of the metal which can release metal ions and fragments into surrounding tissue causing severe adverse events such as:
- • Metallosis – tissue poisoning due to metal ions and fragments
- • Necrosis – tissue and bone death due to metallosis toxicity
- • Osteolysis –dissolution of necrotic bone tissue, caused mainly by metal toxicity
- • Systemic metal poisoning – body wide inflammation due to metallosis entry into the bloodstream
- • Pseudotumors – false tumor formation surrounding the joint due to inflammation
- • Bone fracture – bones near the joint may weaken and fracture
- • Revision Surgery – to replace implant due to pain or severe inflammation
- • Reconstructive surgery – due to weakened bone tissue resulting in fractures of femur or pelvis
Less severe side effects which are not medically dangerous, may impact the patient’s quality of life and ability to perform normal activities. These include:
- • Pain in implant area, groin and abdomen
- • Inflammation in implant area, groin and abdomen
- • Difficulty in standing or walking due to hip instability
- • Loss of muscle mass due to immovable joint
- • Difficulty moving due to pain
- • Hip dislocation
Triathlon Knee Implants
More than 600,000 knee replacements are performed in the US each year

The FDA issued a recall of ShapeMatch Cutting Guide in 2013 due to a software defect, which could display incorrect cutting parameters, resulting in surgeons performing the knee replacement procedure incorrectly.
Ultimately, patients experienced a number of complications with their Triathlon implants, including:
- • Joint instability
- • Joint fracture
- • Chronic pain
- • Swelling and infection
- • Limited mobility or total immobility
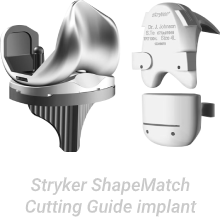
Stryker was forced to negotiate with the Department of Justice for a $33 million settlement.


